Volume 2 Issue 2 2021
EDITORIAL
2021, 2(2): 87–88. doi:https://doi.org/10.12336/biomatertransl.2021.02.001
VIEWPOINT
2021, 2(2): 89–90. doi:https://doi.org/10.12336/biomatertransl.2021.02.002
REVIEW
2021, 2(2): 91–142. doi:https://doi.org/10.12336/biomatertransl.2021.02.003
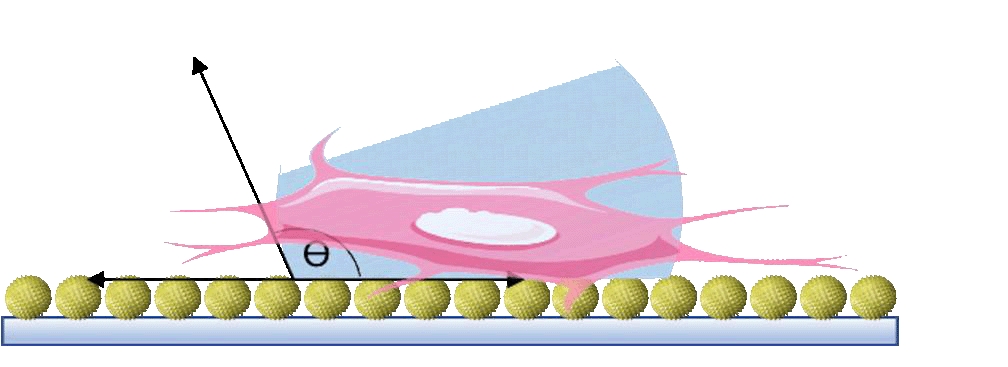
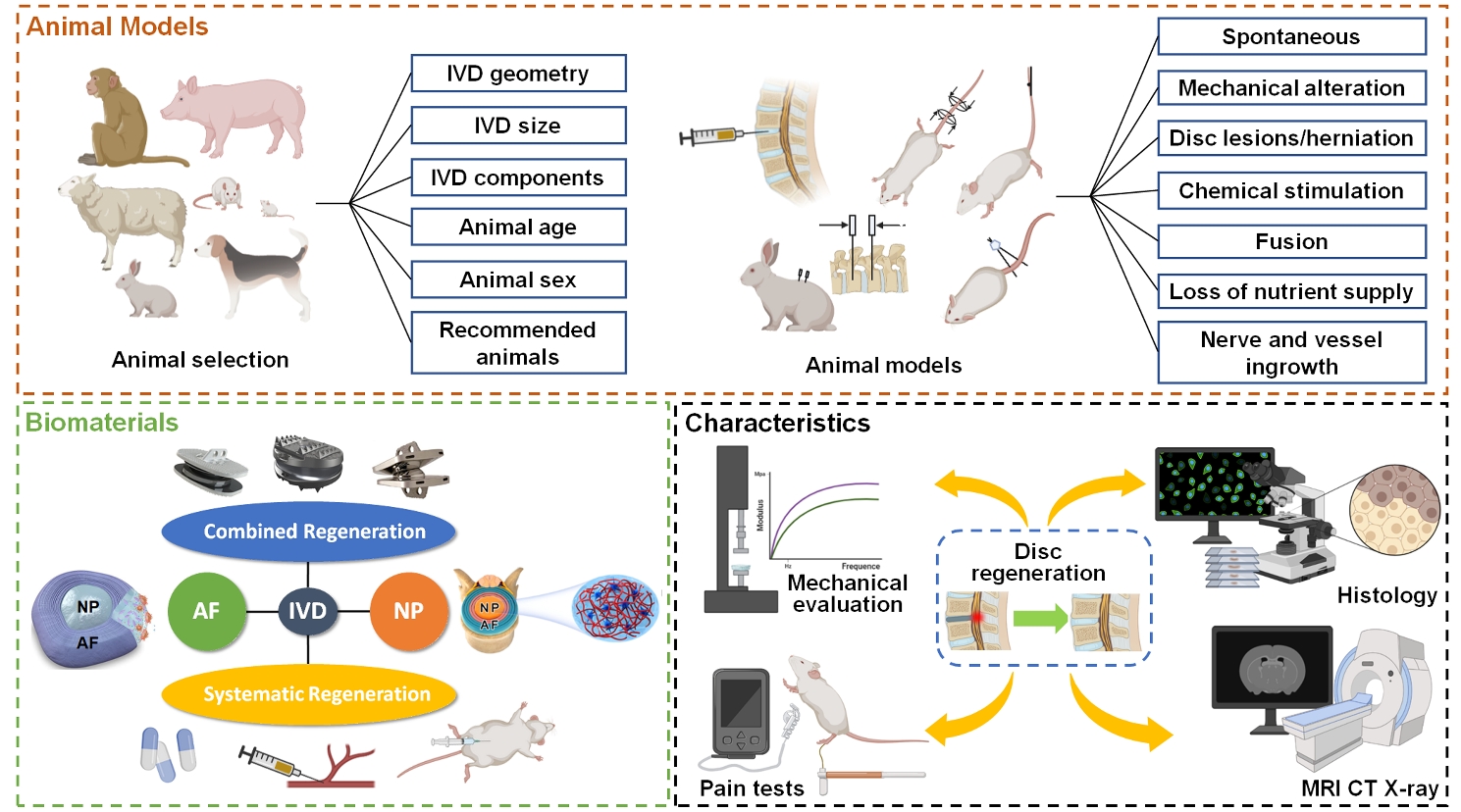
Low back pain is a vital musculoskeletal disease that impairs life quality, leads to disability and imposes heavy economic burden on the society, while it is greatly attributed to intervertebral disc degeneration (IDD). However, the existing treatments, such as medicines, chiropractic adjustments and surgery, cannot achieve ideal disc regeneration. Therefore, advanced bioactive therapies are implemented, including stem cells delivery, bioreagents administration, and implantation of biomaterials etc. Among these researches, few reported unsatisfying regenerative outcomes. However, these advanced therapies have barely achieved successful clinical translation. The main reason for the inconsistency between satisfying preclinical results and poor clinical translation may largely rely on the animal models that cannot actually simulate the human disc degeneration. The inappropriate animal model also leads to difficulties in comparing the efficacies among biomaterials in different reaches. Therefore, animal models that better simulate the clinical charateristics of human IDD should be acknowledged. In addition, in vivo regenerative outcomes should be carefully evaluated to obtain robust results. Nevertheless, many researches neglect certain critical characteristics, such as adhesive properties for biomaterials blocking annulus fibrosus defects and hyperalgesia that is closely related to the clinical manifestations, e.g., low back pain. Herein, in this review, we summarized the animal models established for IDD, and highlighted the proper models and parameters that may result in acknowledged IDD models. Then, we discussed the existing biomaterials for disc regeneration and the characteristics that should be considered for regenerating different parts of discs. Finally, well-established assays and parameters for in vivo disc regeneration are explored.
RESEARCH ARTICLE
2021, 2(2): 143–150. doi:https://doi.org/10.12336/biomatertransl.2021.02.004
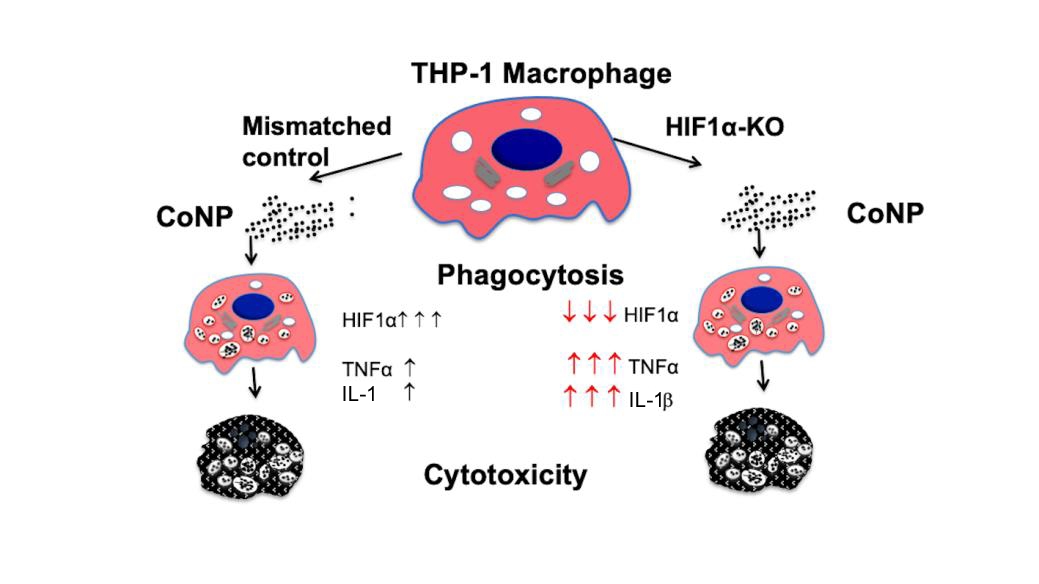
Cobalt is one of the main components of metal hip prostheses and cobalt nanoparticles (CoNPs) produced from wear cause inflammation, bone lyses and cytotoxicity at high concentrations. Cobalt ions mimic hypoxia in the presence of normal oxygen levels, and activate hypoxic signalling by stabilising hypoxia inducible transcription factor 1α (HIF1α). This study aimed to assess in vitro the functional role of HIF1α in CoNP induced cellular cytotoxicity. HIF1α, lysosomal pH, tumour necrosis factor α and interleukin 1β expression were analysed in THP-1 macrophages treated with CoNP (0, 10 and 100 μg/mL). HIF1α knock out assays were performed using small interfering RNA to assess the role of HIF1α in CoNP-induced cytotoxicity. Increasing CoNP concentration increased lysosomal activity and acidity in THP-1 macrophages. Higher doses of CoNP significantly reduced cell viability, stimulated caspase 3 activity and apoptosis. Reducing HIF1α activity increased the pro-inflammatory activity of tumour necrosis factor α and interleukin 1β, but had no significant impact on cellular cytotoxicity. This suggests that whilst CoNP promotes cytotoxicity and cellular inflammation, the apoptotic mechanism is not dependent on HIF1α.
RESEARCH ARTICLE
2021, 2(2): 151–164. doi:https://doi.org/10.12336/biomatertransl.2021.02.005
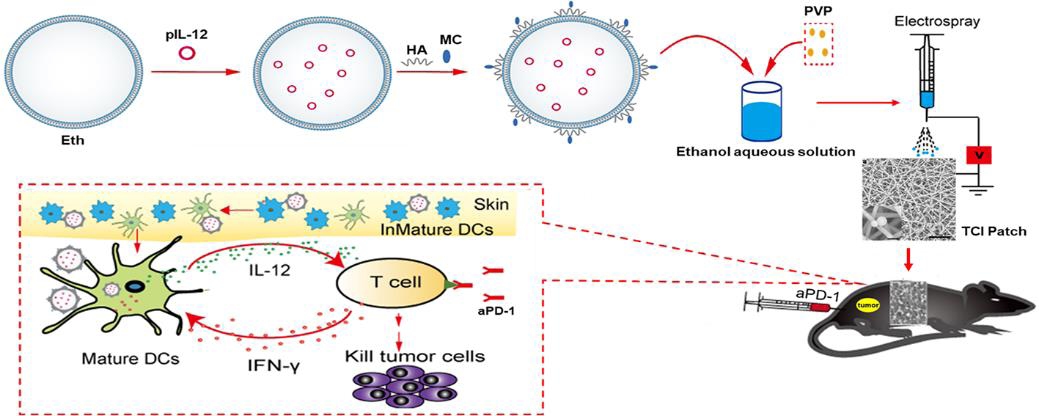
Recent studies have suggested that the anti-tumour effect of the programmed cell death protein 1 monoclonal antibody (aPD-1) depends on the expression of interleukin-12 (IL-12) by dendritic cells (DCs). Since DCs are abundant in skin tissues, transdermal delivery of IL-12 targeting DCs may significantly improve the anti-tumour effect of aPD-1. In this study, a novel mannosylated chitosan (MC)-modified ethosome (Eth-MC) was obtained through electrostatic adsorption. The Eth-MC loaded with plasmid containing the IL-12 gene (pIL-12@Eth-MC) stimulated DCs to express mature-related molecular markers such as CD86, CD80, and major histocompatibility complex-II in a targeted manner. The pIL-12@Eth-MC was then mixed with polyvinyl pyrrolidone solution to make microspheres using the electrospray technique, and sprayed onto the surface of electrospun silk fibroin-polyvinyl alcohol nanofibres to obtain a PVP-pIL-12@Eth-MC/silk fibroin-polyvinyl alcohol composite nanofibrous patch (termed a transcutaneous immunization (TCI) patch). The TCI patch showed a good performance on transdermal drug release. Animal experiments on melanoma-bearing mice showed that topical application of the TCI patches promoted the expression of IL-12 and inhibited the growth of tumour. Furthermore, combined application of the TCI patch and aPD-1 showed a stronger anti-tumour effect than aPD-1 monotherapy. The combination therapy significantly promoted the expression of IL-12, interferon-γ and tumour necrosis factor-α, the infiltration of CD4+ and CD8+ T cells into tumour tissues, and thus promoted the apoptosis of tumour cells. The present study provides a convenient and non-invasive strategy for improving the efficacy of immune checkpoint inhibitor therapy. This study was approved by the Institutional Animal Care and Use Committee at Donghua University (approval No. DHUEC-NSFC-2020-11) on March 31, 2020.
RESEARCH ARTICLE
2021, 2(2): 165–173. doi:https://doi.org/10.12336/biomatertransl.2021.02.006
The surface free energy of a biomaterial plays an important role in the early stages of cell-biomaterial interactions, profoundly influencing protein adsorption, interfacial water accessibility, and cell attachment on the biomaterial surface. Although multiple approaches have been developed to engineer the surface free energy of biomaterials, systematically tuning their surface free energy without altering other physicochemical properties remains challenging. In this study, we constructed an array of chemically-equivalent surfaces with comparable apparent roughness through assembly of gold nanoparticles adopting various geometrically-distinct shapes but all capped with the same surface ligand, (1-hexadecyl)trimethylammonium chloride, on cell culture substrates. We found that bone marrow stem cells exhibited distinct osteogenic differentiation behaviours when interacting with different types of substrates comprising shape-controlled gold nanoparticles. Our results reveal that bone marrow stem cells are capable of sensing differences in the nanoscale topographical features, which underscores the role of the surface free energy of nanostructured biomaterials in regulating cell responses. The study was approved by Institutional Animal Care and Use Committee, School of Medicine, University of South Carolina.