News and Announcements
Volume 3 Issue 3 2022
Order by
Latest time
EDITORIAL
A milestone towards a successful scientific journal: celebrating the inclusion of Biomaterials Translational by PubMed
Qian Wang
3 Download 824 Views
REVIEW
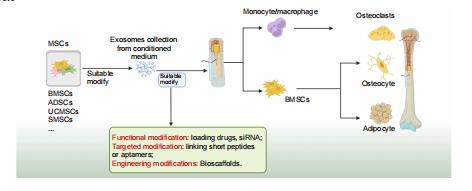
Mesenchymal stem cell–derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review
Zhao–Lin Zeng,
Hui Xie
10 Download 1756 Views
REVIEW
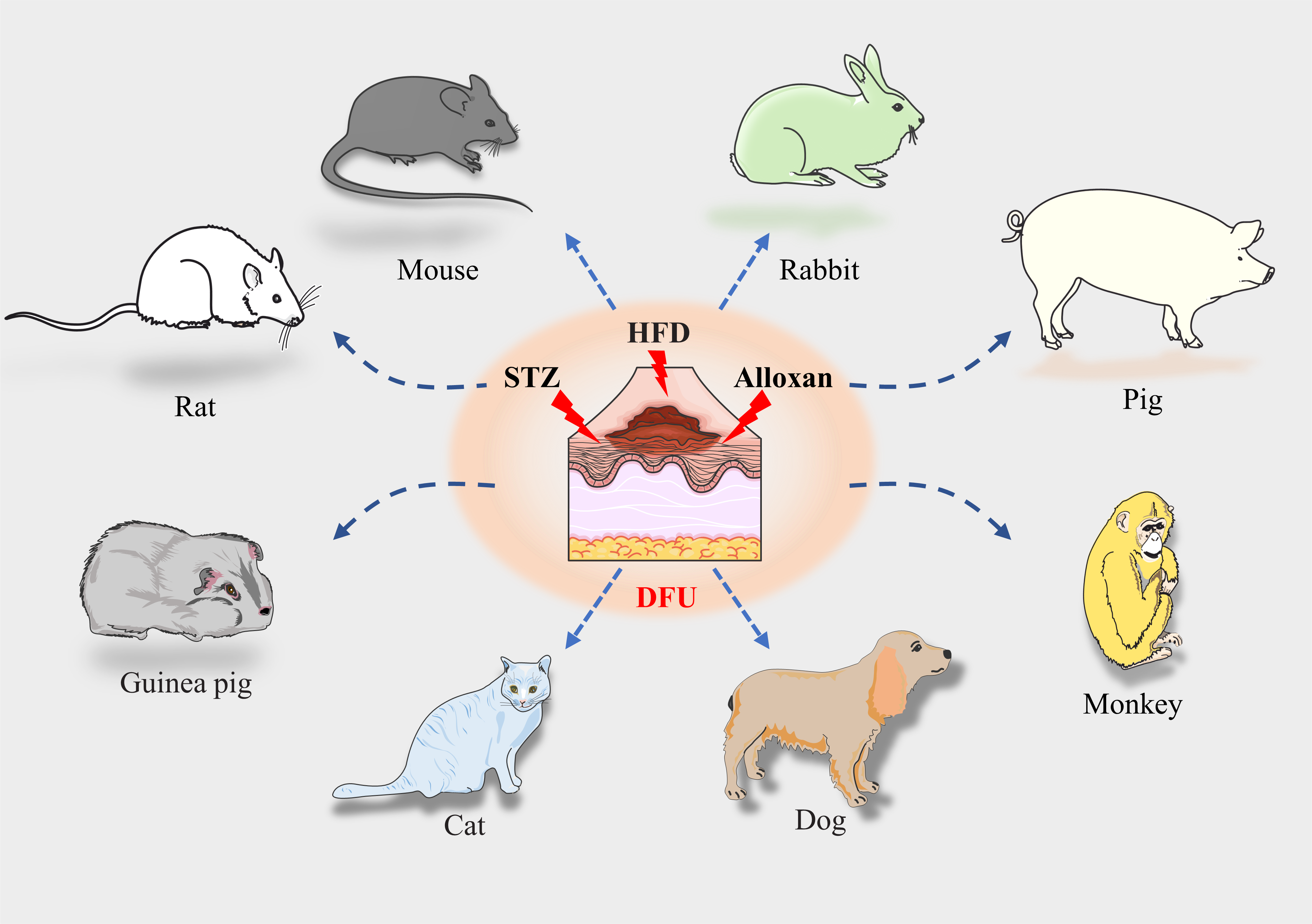
Advances and perspective on animal models and hydrogel biomaterials for diabetic wound healing
Yiqiang Hu,
Yuan Xiong,
Ranyang Tao,
Hang Xue, ... Guohui Liu
65 Download 2983 Views
REVIEW
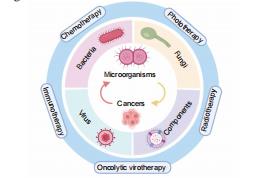
Engineered microorganism–based delivery systems for targeted cancer therapy: a narrative review
Xin Huang,
Haoyu Guo,
Lutong Wang,
Zengwu Shao
11 Download 1483 Views
RESEARCH ARTICLE
Osteogenic differentiation of encapsulated cells in dexamethasone–loaded phospholipid–induced silk fibroin hydrogels
Chavee Laomeephol,
Helena Ferreira,
Sorada Kanokpanont,
Jittima Amie Luckanagul, ... Siriporn Damrongsakkul
13 Download 1073 Views
RESEARCH ARTICLE
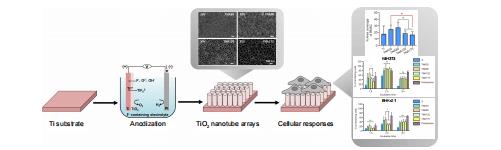
Cellular responses to nanoscale substrate topography of TiO2 nanotube arrays: cell morphology and adhesion
Monchupa Kingsak,
Panita Maturavongsadit,
Hong Jiang,
Qian Wang
18 Download 1185 Views