Advancing biomedical innovation through composite material strategies
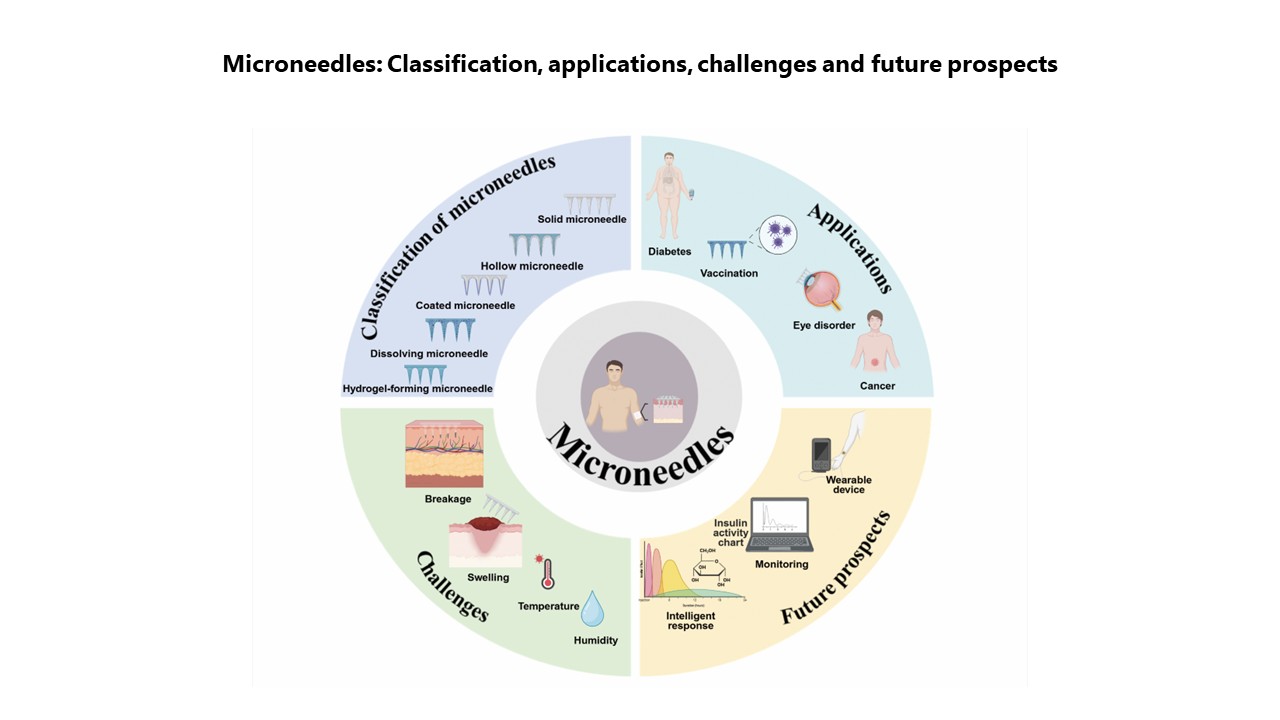
Microneedles in biomedicine: Innovations, challenges, and future prospects
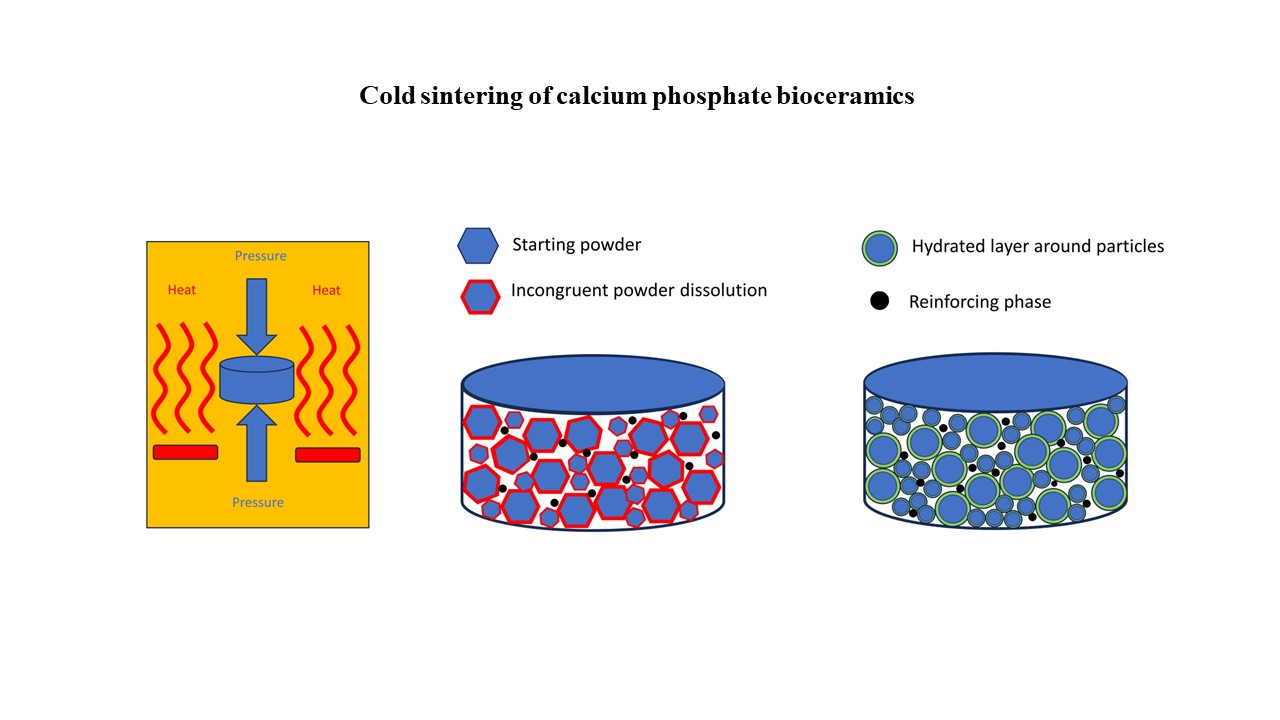
Cold-sintered bioceramics for medical applications: State of the art and further perspectives
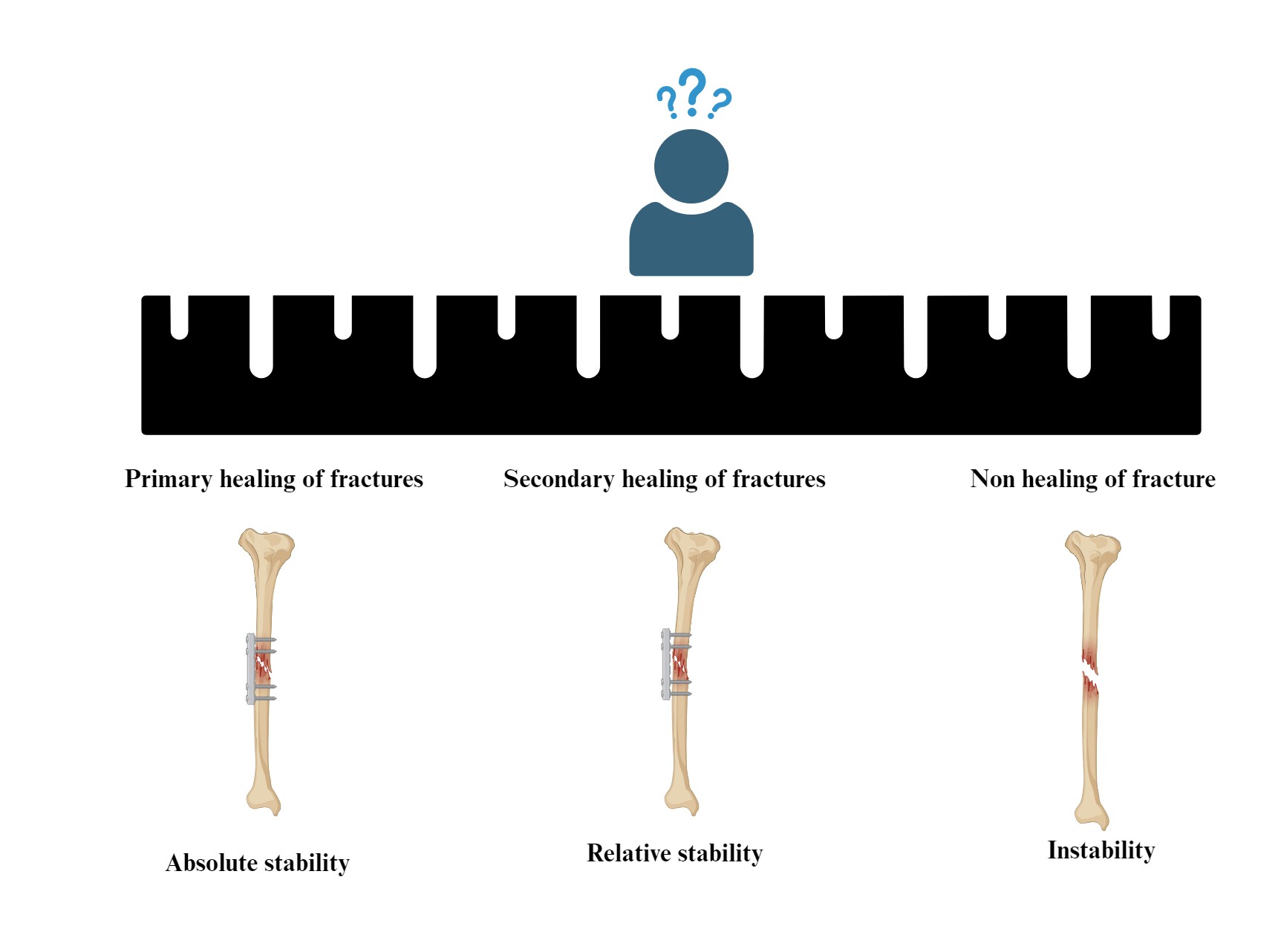
An exploration into the principles and theoretical progress of fracture treatment based on the mechanism of fracture healing
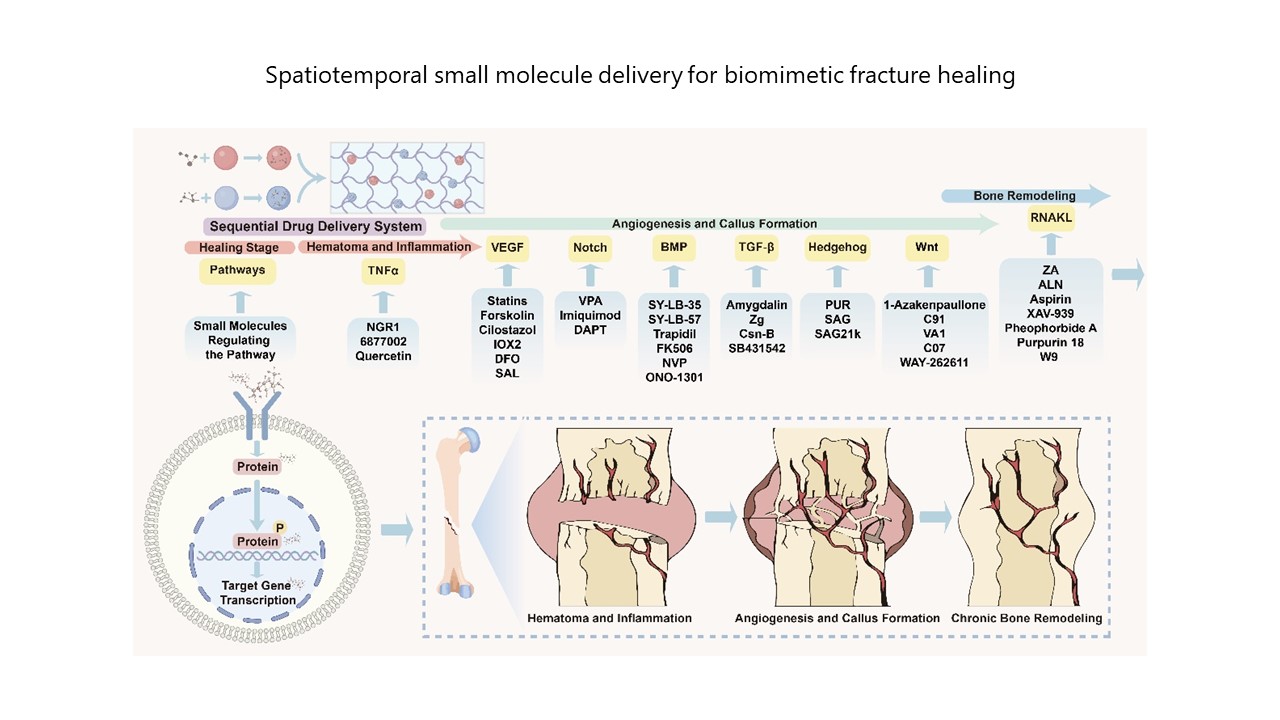
Spatiotemporal application of small molecules in fracture healing
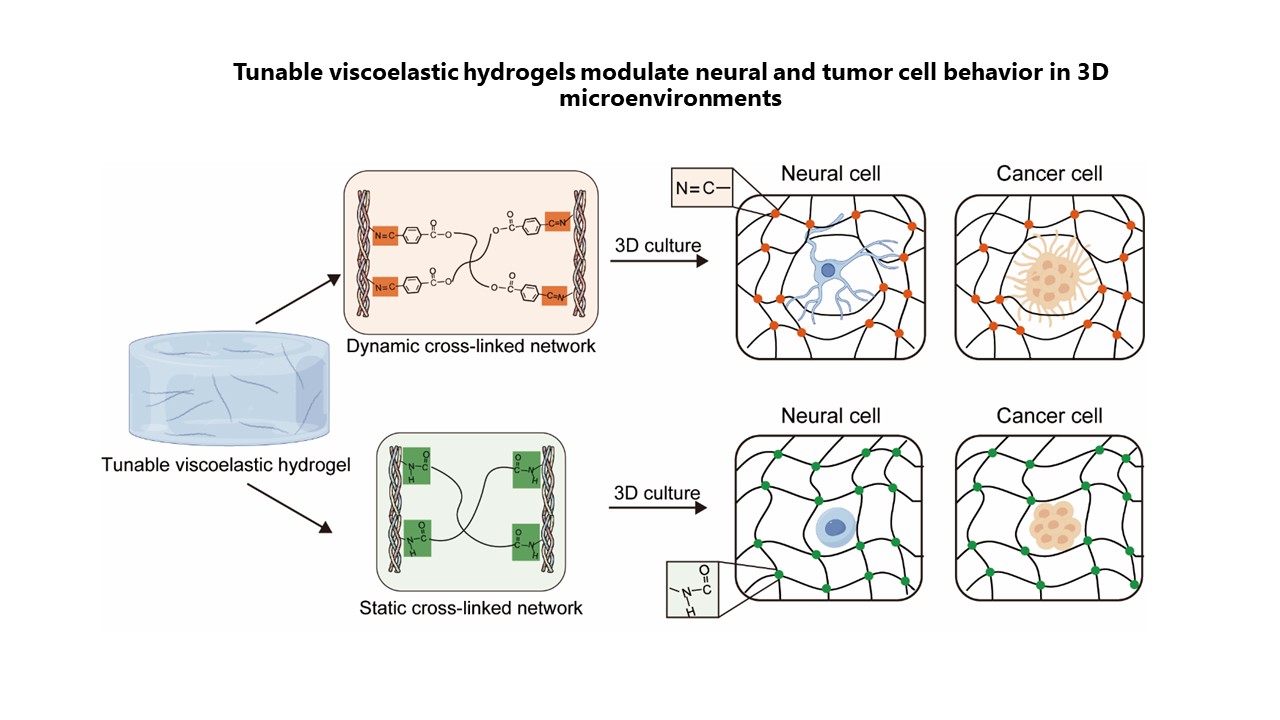
Tunable viscoelastic collagen/polyethylene glycol composite hydrogels modulate neural and tumor cell behavior in 3D microenvironments
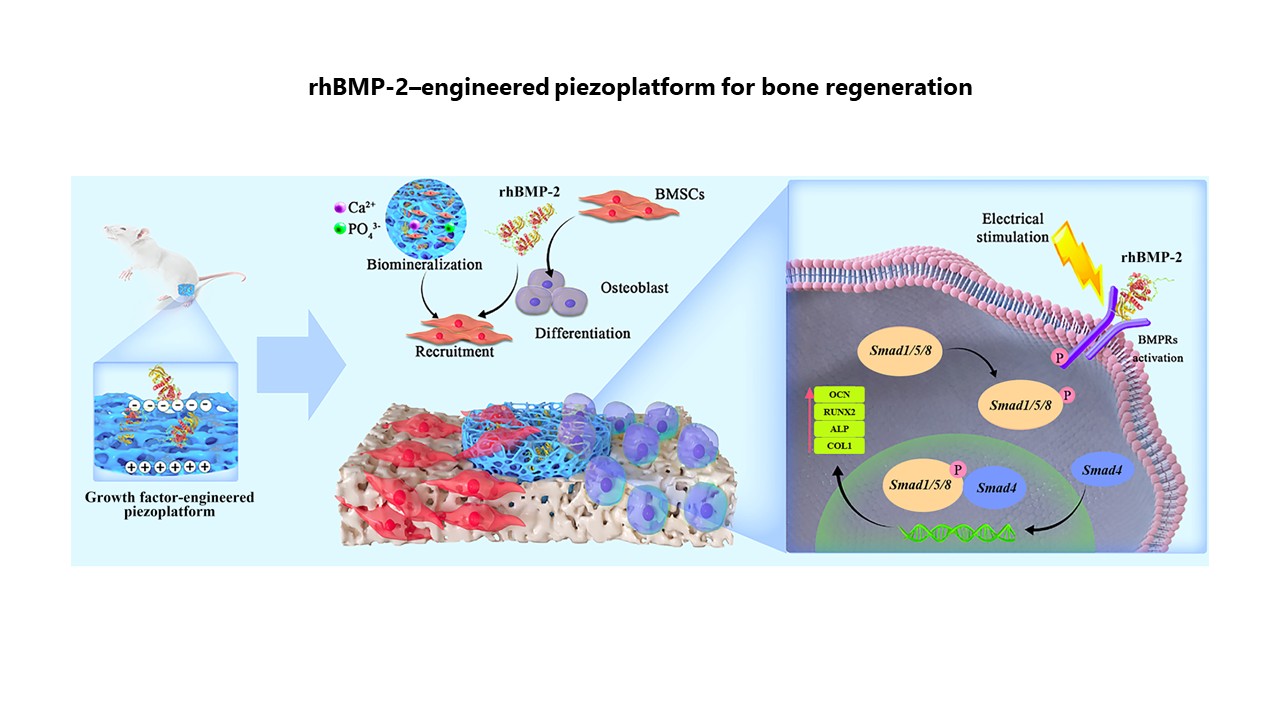
Recombinant human bone morphogenetic protein-2–engineered piezoplatform synergistically promotes bone regeneration through bone morphogenetic protein receptor activation
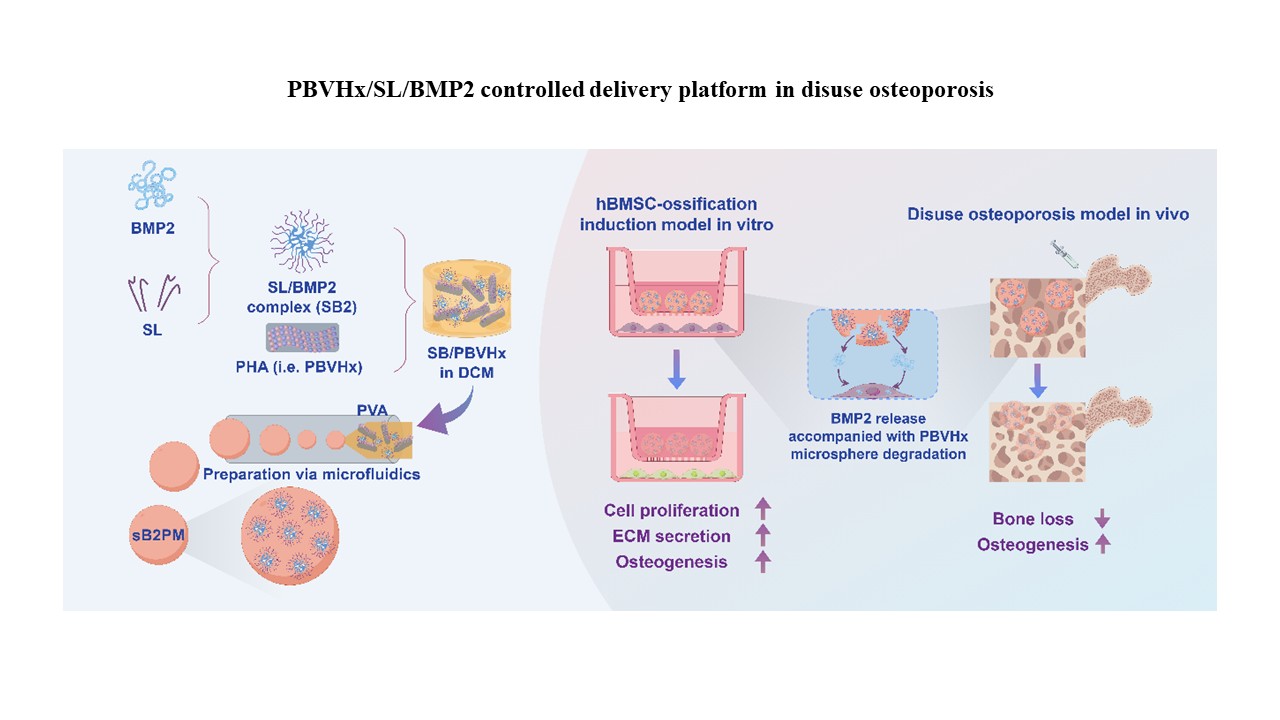
PBVHx-based microspheres for controlled BMP2 release and enhanced bone regeneration in a disuse osteoporosis mouse model
Integrating nanomedicine and immunotherapy: Bacterial membrane–derived vesicle-encapsulated prodrug assemblies for chronic infections
The advancement of composite materials in future biomedical technologies



