News and Announcements
Volume 3 Issue 4 2022
Order by
Download Full Issue
Latest time
EDITORIAL
Celebrating the 2nd anniversary of Biomaterials Translational
Zhidao Xia,
Qian Wang
2 Download 914 Views
VIEWPOINT
Skeletal interoception: an emerging area for musculoskeletal research
Zhidao Xia
5 Download 960 Views
VIEWPOINT
Engineered exosomes for future gene-editing therapy
Haoyu Guo,
Xin Huang
11 Download 1107 Views
RESEARCH ARTICLE
Optimising soft tissue in-growth in vivo in additive layer manufactured osseointegrated transcutaneous implants
Elena Giusto,
Gordon Blunn,
Roberta Ferro de Godoy,
Chaozong Liu,
Catherine Pendegrass
4 Download 1022 Views
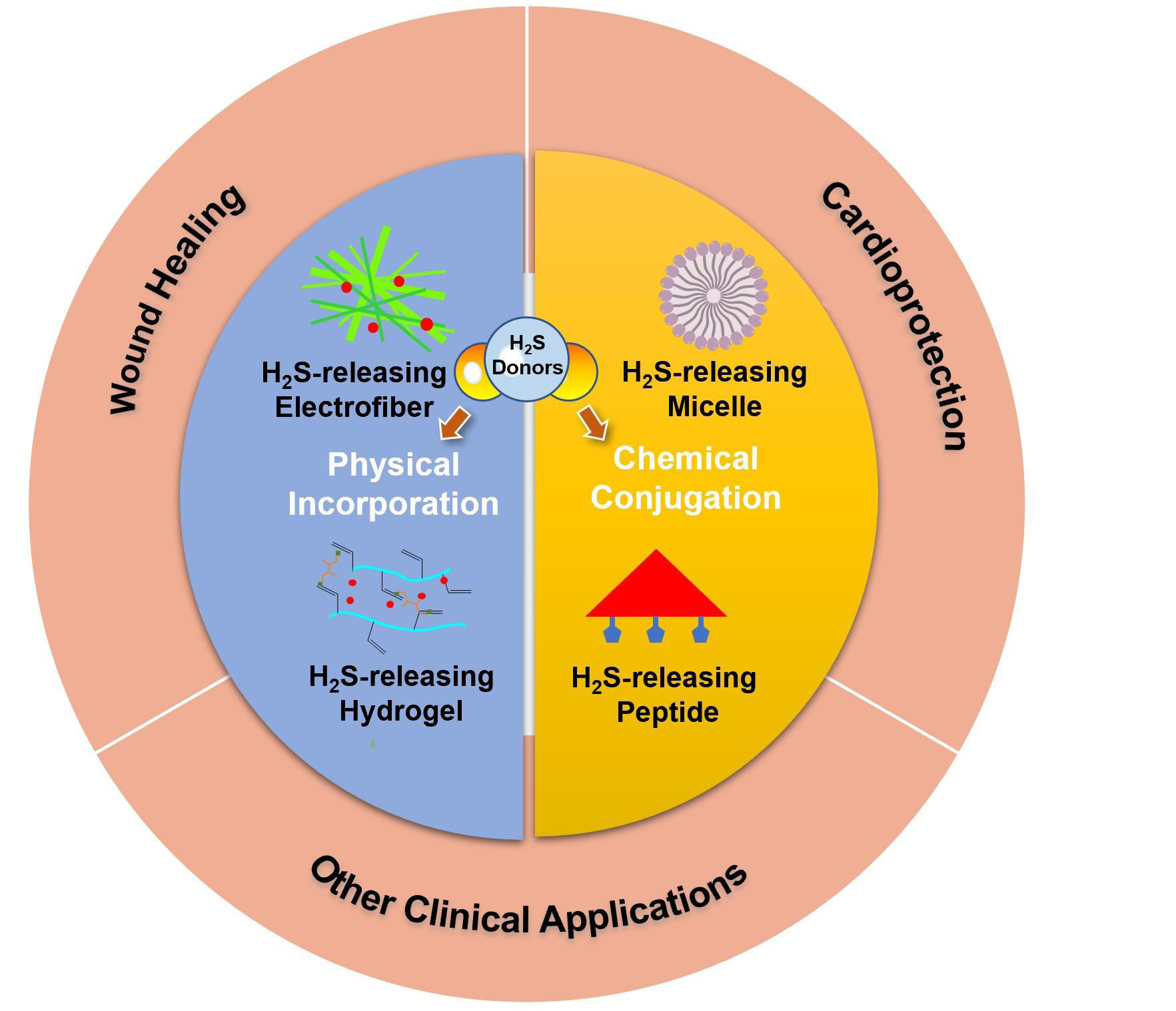
REVIEW
Recent development of hydrogen sulfide-releasing biomaterials as novel therapies:a narrative review
Jingyu Fan,
Elizabeth Pung,
Yuan Lin,
Qian Wang
22 Download 1292 Views
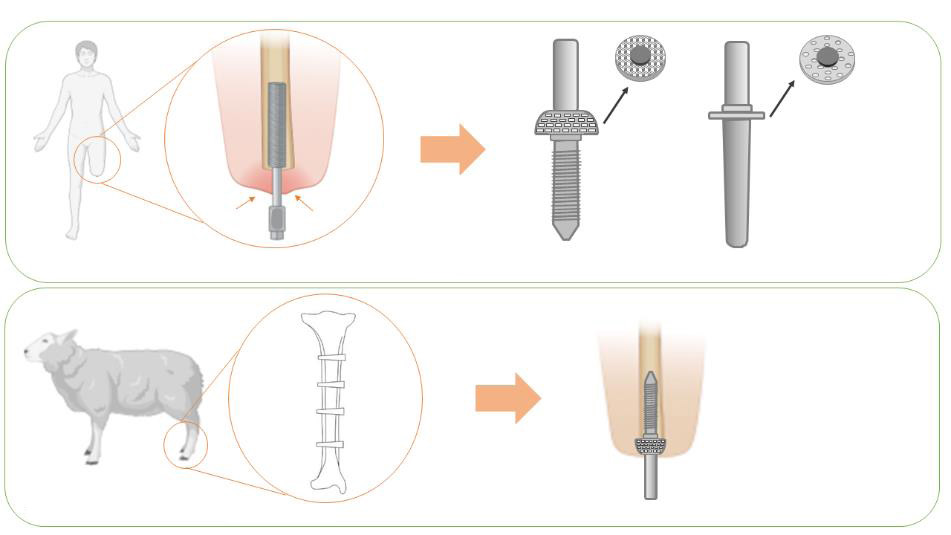
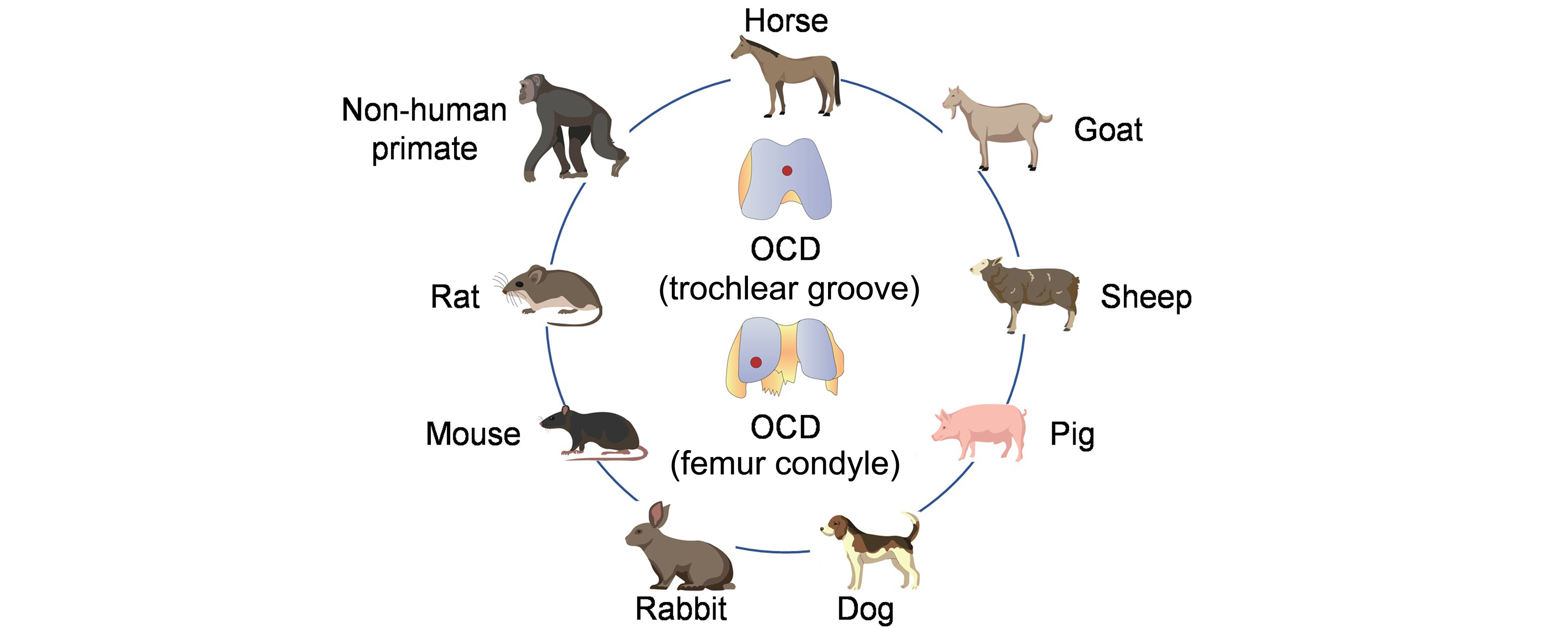
REVIEW
Osteoarthritis animal models for biomaterial-assisted osteochondral regeneration
Yi Wang,
Yangyang Chen,
Yulong Wei
18 Download 1923 Views
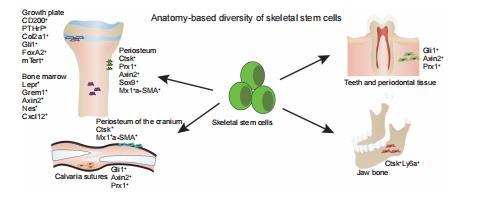
REVIEW
New perspective of skeletal stem cells
Guixin Yuan,
Zan Li,
Xixi Lin,
Na Li,
Ren Xu
10 Download 1240 Views