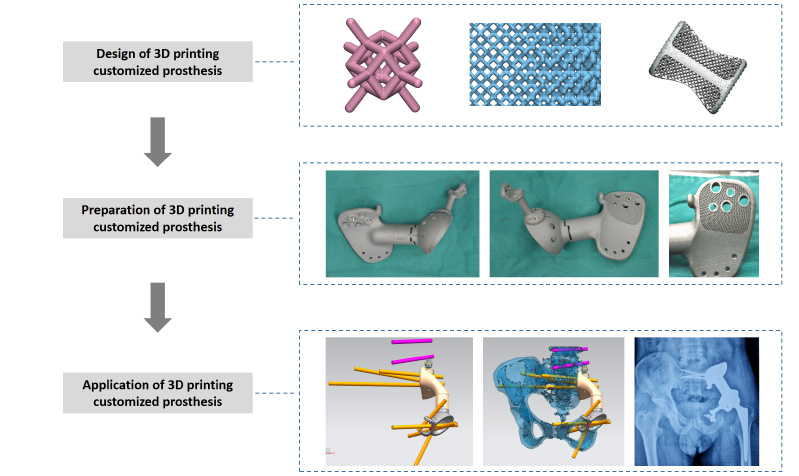
Additive manufacturing innovation for musculoskeletal tissue repair and regeneration: from bench to bedside
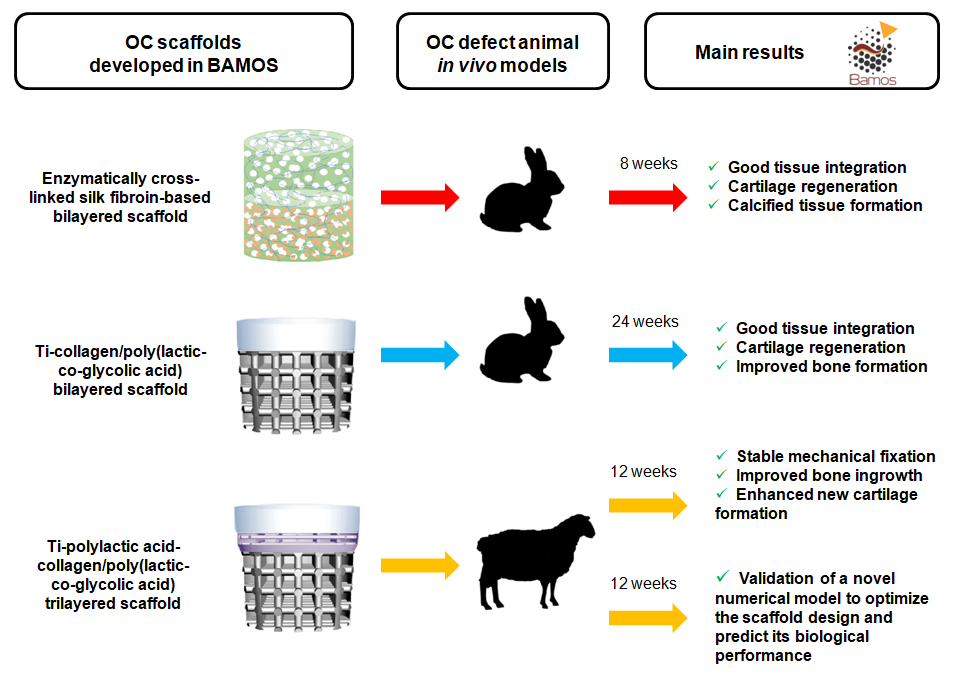
Translation through collaboration: practice applied in BAMOS project in in vivo testing of innovative osteochondral scaffolds
Three-dimensional bio-printing of decellularized extracellular matrix-based bio-inks for cartilage regeneration: a systematic review
Additive manufactured polyether-ether-ketone implants for orthopaedic applications: a narrative review
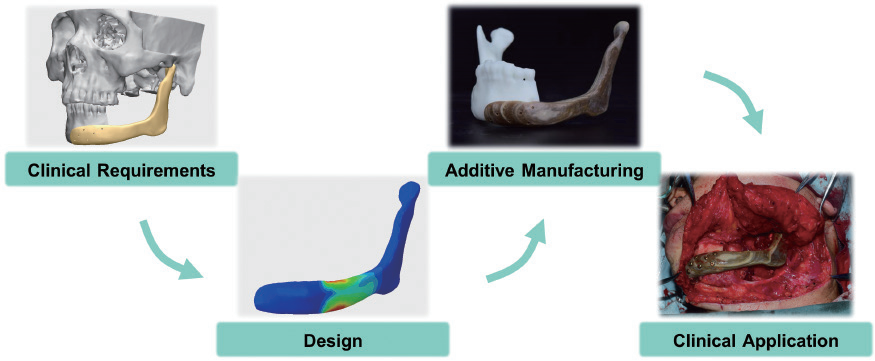
Three-dimensional-printed titanium prostheses with bone trabeculae enable mechanical-biological reconstruction after resection of bone tumours
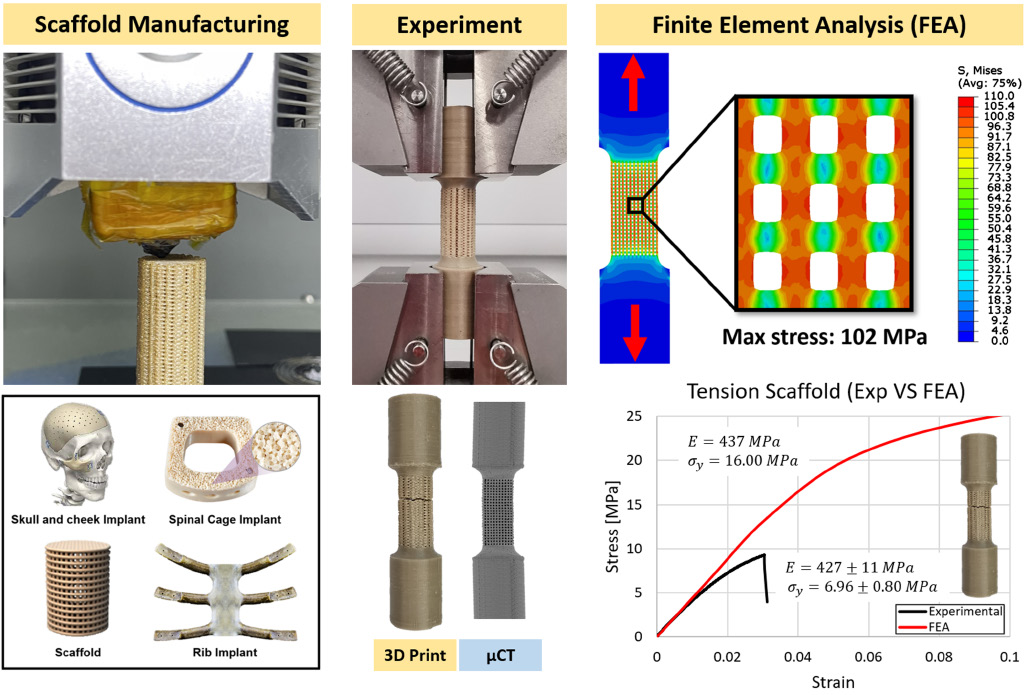
On the mechanical aspect of additive manufactured polyether-ether-ketone scaffold for repair of large bone defects
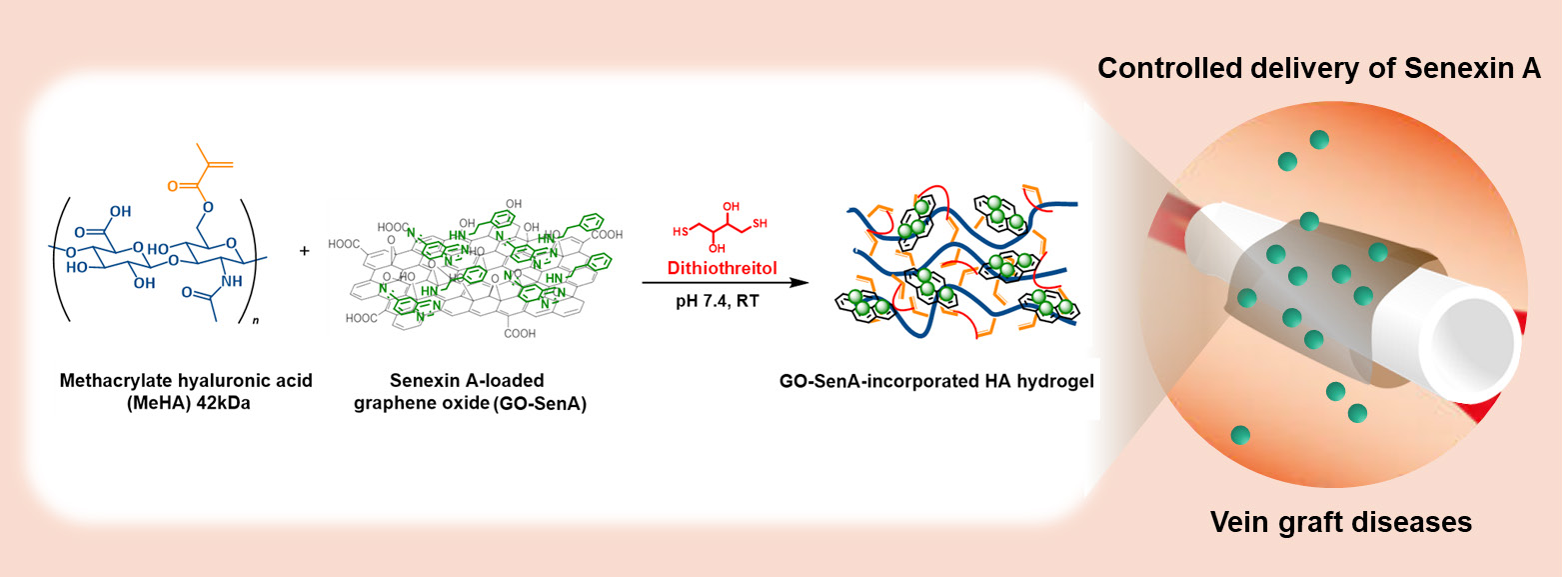
Graphene-incorporated hyaluronic acid-based hydrogel as a controlled Senexin A delivery system
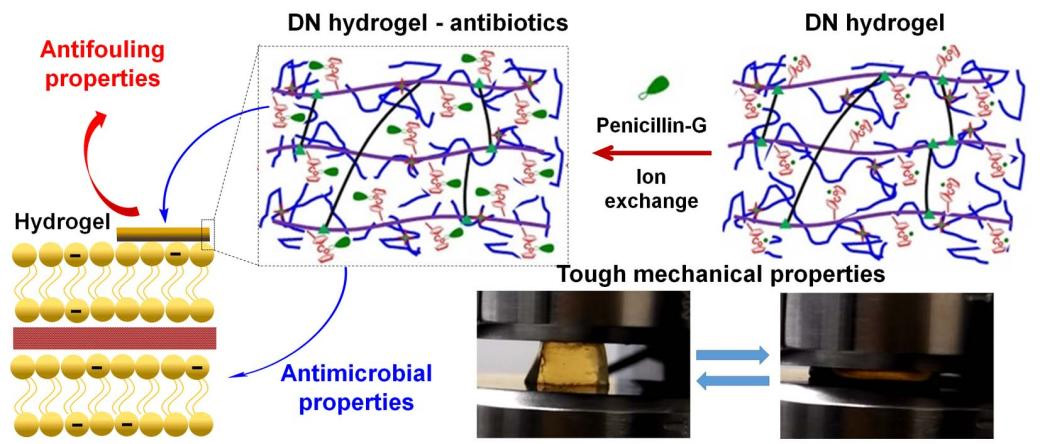
Antifouling and antimicrobial cobaltocenium-containing metallopolymer double-network hydrogels