News and Announcements
Volume 5 Issue 3 2024
Order by
Download Full Issue
Latest time
EDITORIAL
Advances and challenges in hydrogel microspheres for biomedical applications
Yiting Lei,
Hélder A Santos,
Wenguo Cui
75 Download 2074 Views
REVIEW
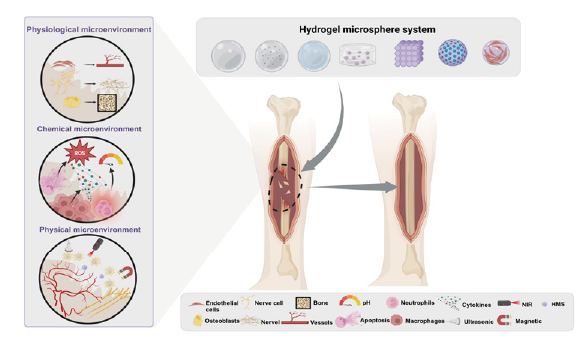
Hydrogel microspheres for bone regeneration through regulation of the regenerative microenvironment
Pengrui Zhang,
Qiwei Qin,
Xinna Cao,
Honglin Xiang, ... Yuling Li
124 Download 5052 Views
REVIEW
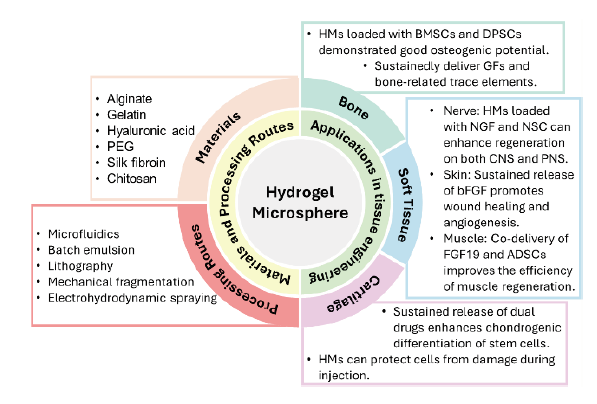
The use of hydrogel microspheres as cell and drug delivery carriers for bone, cartilage, and soft tissue regeneration
Chung-Hsun Lin,
Jesse R. Srioudom,
Wei Sun,
Malcolm Xing, ... Jian Yang
99 Download 3713 Views
REVIEW
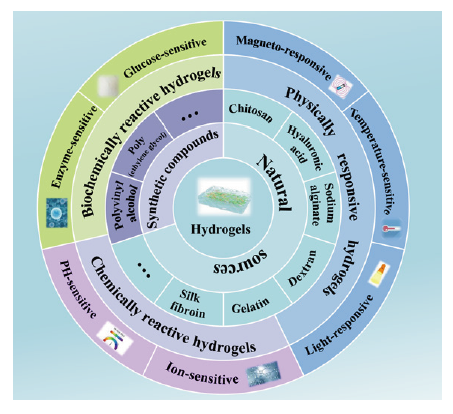
Stimuli-responsive hydrogels for bone tissue engineering
Congyang Xue,
Liping Chen,
Nan Wang,
Heng Chen, ... Xin Liu
65 Download 2943 Views
REVIEW
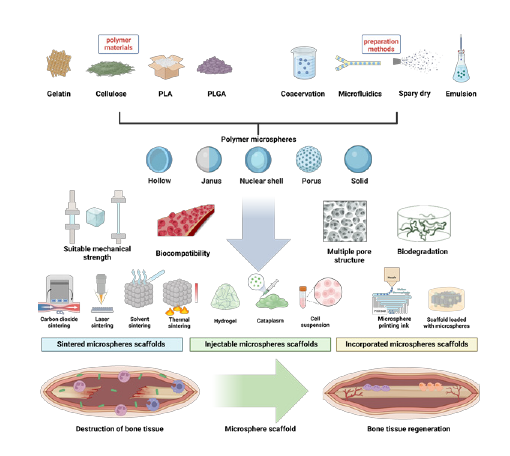
From the microspheres to scaffolds: advances in polymer microsphere scaffolds for bone regeneration applications
Shuhao Yang,
Haoming Wu,
Chao Peng,
Jian He, ... Xulin Hu
59 Download 2422 Views
RESEARCH ARTICLE
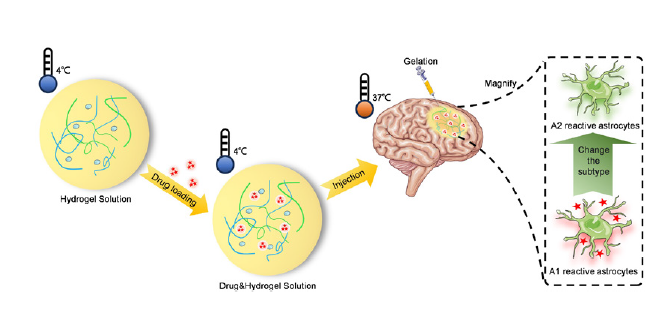
Injectable body temperature responsive hydrogel for encephalitis treatment via sustained release of nano-anti-inflammatory agents
Yuqi Gai,
Huaijuan Zhou,
Yingting Yang,
Jiatian Chen, ... Jinhua Li
130 Download 1886 Views
RESEARCH ARTICLE
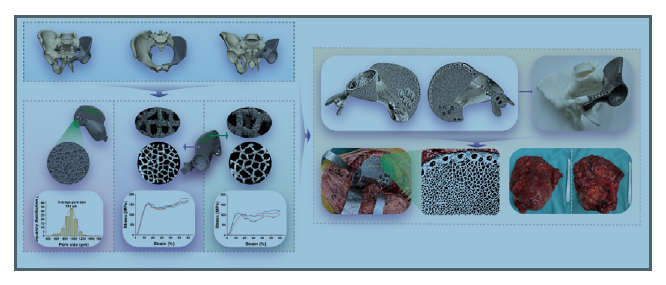
Design, characterisation, and clinical evaluation of a novel porous Ti-6Al-4V hemipelvic prosthesis based on Voronoi diagram
Zhuangzhuang Li,
Yi Luo,
Minxun Lu,
Yitian Wang, ... Chongqi Tu
61 Download 1619 Views
COMMENTARY
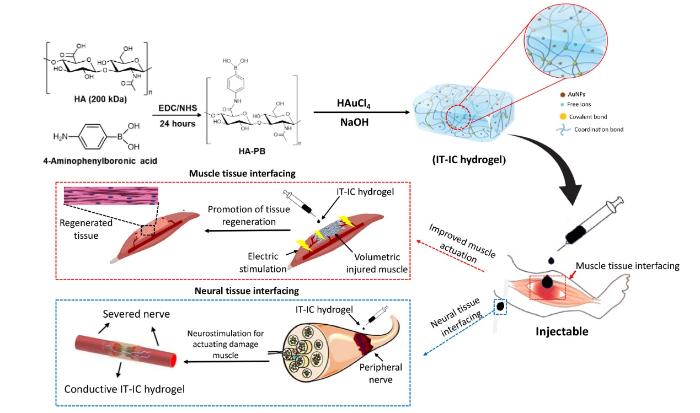
Bio-instructive hydrogel as an injectable tissue prosthesis for the repair and rehabilitation of impaired muscle
Muhammad Arif,
Tengbo Yu,
Qihui Zhou
25 Download 1008 Views
COMMENTARY
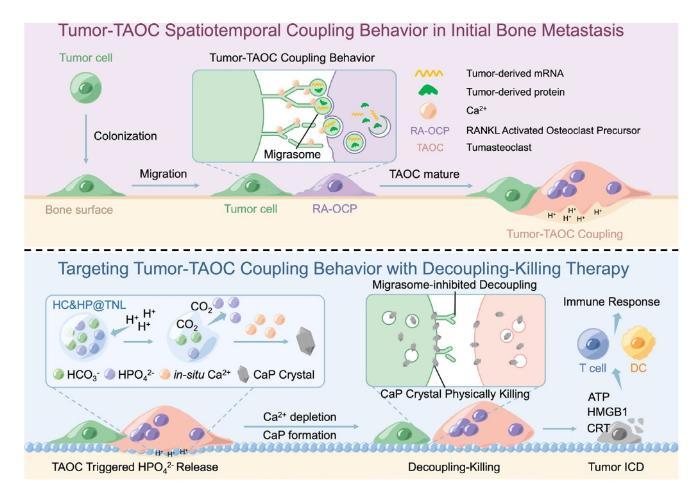
Targeting tumour-osteoclast interactions: a trigger-explosion system to combat bone metastasis
Ang Gao,
Huaiyu Wang
18 Download 1080 Views
COMMENTARY
The potential of three-dimensional printed stents in post-operative treatment of breast cancer
Junjuan Fan,
Min Wang,
Xianwen Wang
18 Download 1086 Views